CTLA-4 Program
Gotistobart, the next generation of CTLA-4 antibodies
What is CTLA-4?
Current limitations of anti-CTLA-4 immunotherapy
In currently approved treatments that exploit the CTLA-4 pathway, CTLA-4 degrades once dragged into lysosome in the T-cells by anti-CTLA-4 antibodies. This means that the CTLA-4 proteins are no longer available for effectively targeting regulatory T cells by anti-CTLA4 therapies, reducing clinical activities of the anti-CTLA-4 antibodies. At the same time, degradation of the CTLA-4 immune-tolerance checkpoint by the currently approved antibodies increased their toxicity.
Our team identified that the differential pH sensitivity of anti–CTLA-4 antibodies to the extracellular versus intracellular environment is critical to preserving CTLA-4 protein—a valuable insight gained during the development of gotistobart.
The gotistobart edge
As a pH-sensitive monoclonal antibody (mAb), our candidate allows for CTLA-4 proteins to be recycled.
After gotistobart binds to the CTLA-4 receptor at the cell surface, the complex is internalized. As a result of the pH change, the mAb unbinds and allows the CTLA-4 protein to return to the surface and continue its role in anticancer signaling.
In effect, gotistobart was designed to selectively deplete the tumor-infiltrating regulatory T cells by preserving CTLA-4 recycling.1 In the peripheral tissues, preserving CTLA-4 recycling also preserves its ability to control autoreactive T cells, thus reducing IrAEs.
1. Zhang Y, Du X, Liu M, Tang F, Zhang P, Ai C, Fields JK, Sundberg EJ, Latinovic OS, Devenport M, Zheng P, Liu Y. Hijacking antibody-induced CTLA-4 lysosomal degradation for safer and more effective cancer immunotherapy. Cell Res 2019 Aug;29(8):609-627. doi: 10.1038/s41422-019-0184-1
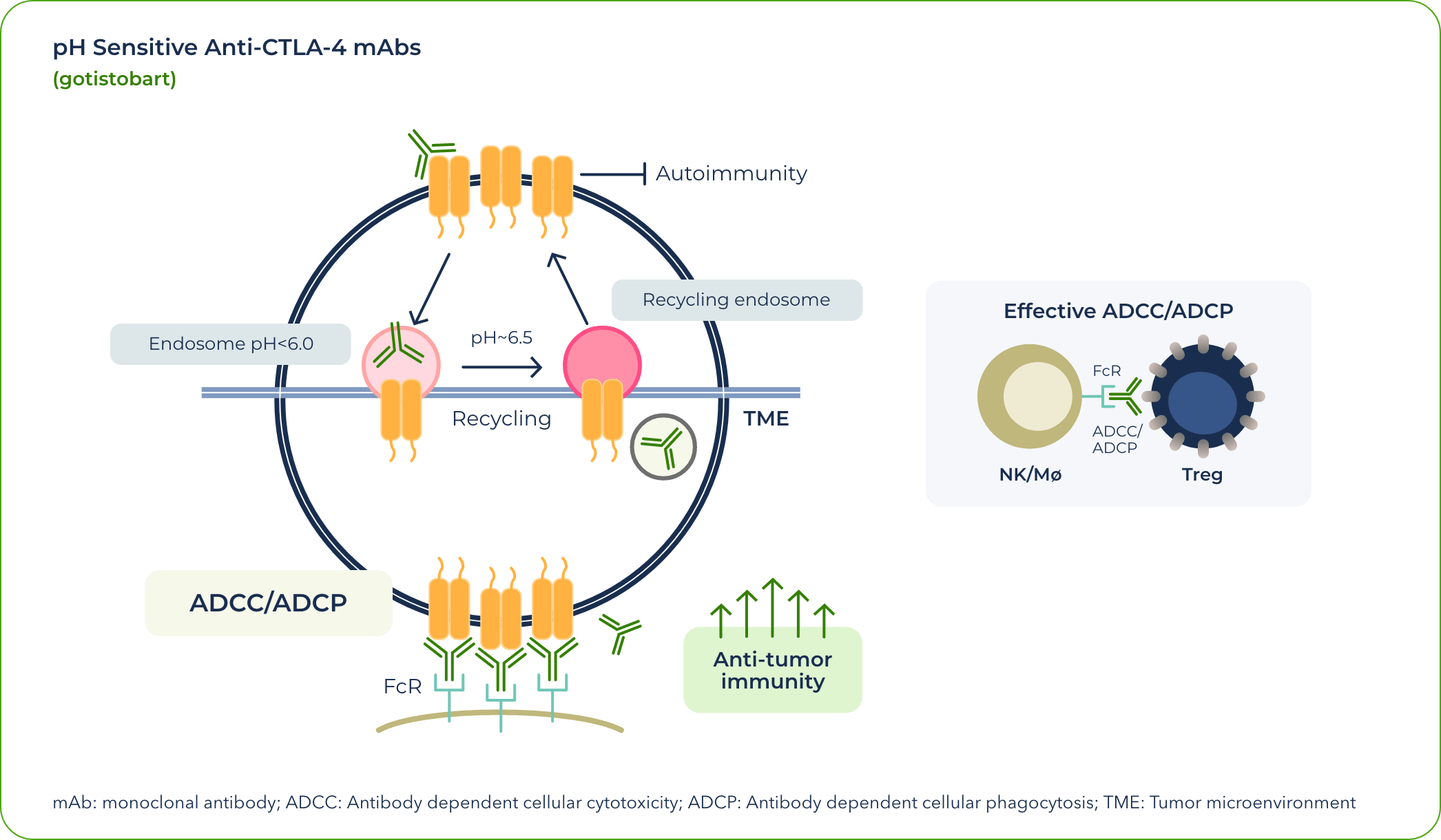
The increased density of CTLA-4 offers a range of potential benefits, including:
An improved safety profile versus the current standard of care, which allows for higher and longer exposure
Reduced toxicity, as shown in in vivo models
Better suited for combination therapy
In clinical trials
As a next-generation anti-CTLA-4 antibody, gotistobart is being co-developed clinically by BioNTech and OncoC4.
Gotistobart is currently being evaluated in an ongoing Phase 1/2 trial, PRESERVE-001, (NCT04140526) in patients with advanced solid tumors as a single agent or in combination with pembrolizumab, and an ongoing registrational Phase 3 trial, PRESERVE-003 (NCT05671510) evaluates the candidate as monotherapy in patients with metastatic, immunotherapy-resistant non-small cell lung cancer (NSCLC).
Gotistobart showed meaningful clinical activity in patients with solid tumors and in those with metastatic non–small cell lung cancer without driver mutations whose disease had progressed on prior lines of immunotherapy-based regimens.
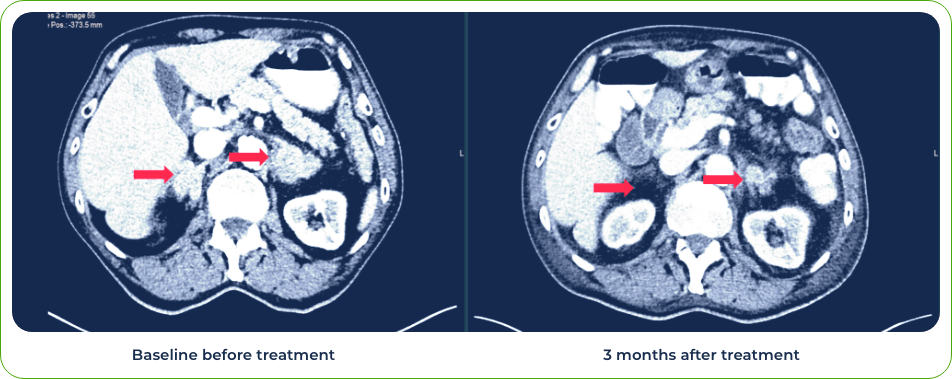
Case 1: 75-year-old male treated with gotistobart
Diagnosis:
Stage IV lung adenosquamous carcinoma
Sites of metastases:
Adrenal glands and brain
Case 2: 64-year-old male treated with gotistobart
Diagnosis:
Squamous cell carcinoma of lung
Sites of metastases:
Spleen and liver
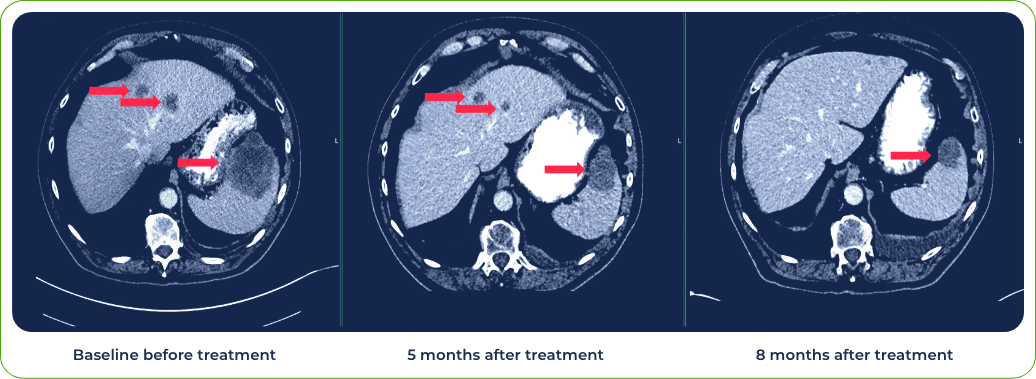
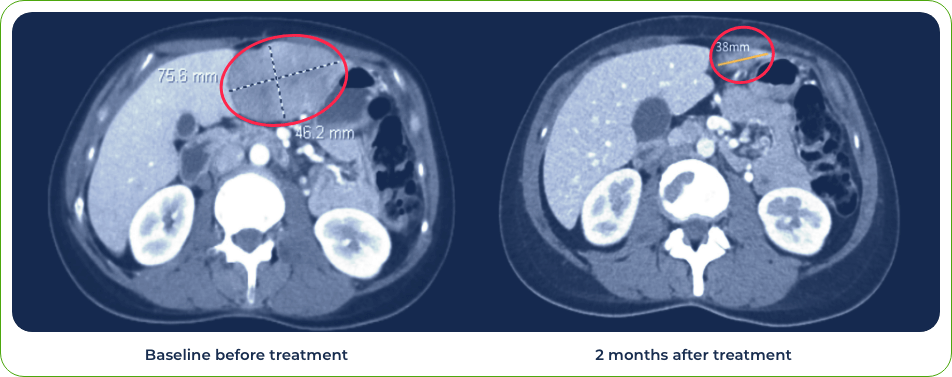
Case 3: Patient treated with combination of gotistobart and pembrolizumab
Single dose induced regression of large metastatic lesion in the liver
Combination potential
As a pH-sensitive monoclonal antibody (mAb), our candidate allows for CTLA-4 proteins to be recycled.
PD-1 is another protein that acts as an “off switch” for T cells, helping to regulate the immune system. PD-1 inhibitors are often given in combination with other checkpoint inhibitors.
We are currently evaluating gotistobart in a Phase 2 trial as a combination therapy with pembrolizumab in platinum-resistant ovarian cancer (NCT05446298). It is also being evaluated in the PRESERVE-006 (NCT05682443) Phase 2 trial in combination with PLUVICTO® (lutetium Lu 177 vipivotide tetraxetan) in patients with metastatic castration resistant prostate cancer who have progressed on androgen receptor pathway inhibitors and the chemotherapy taxane agents.
See how we turn our science into action.
AI-061
1:1 fixed dose in the same buffer
Coformulation has the potential to:
- Expand gotistobart patent
- Reduce cost of combination treatments
- Reduce burden of administration of combination treatments
- Clinical monotherapy safety data available
- Higher affinity and better efficacy vs pembrolizumab in preclinical models